ThermoHub represents a hub for storing various type of thermodynamic data and thermodynamic datasets (databases), built using state of the art data storage and management tools.
-
Uses a flexible data format which allows storage of different type of data, each data record being independent of each other. The format allows changes to be added later, additional fields in the data that does not affect the already stored data.
-
Has the capabilities of a graph database, useful in maintaining internal consistency and data traceability. A graph layer can be independently added on top of the individual data types, representing different links between records (e.g. links to data source records).
ThermoHub graph database is managed with ThermoMatch
ThermoHubClient can be used to retrieve thermodynamic data sets from ThermoHub.
Storage of Structured Data
- Local or remote (cloud) storage
Access directly the most recent and updated data (in the cloud); user access
- Data stored in a flexible structured format
Chemical systems can be represented using hierarchically structured data (JSON, YAML, XML) much easier than using plain tables Allows storage of diverse data with different structures In a JSON document, only fields with existing data are present New data fields can be added any time without affecting the existing data
Graph Database
- On top of individual data types (e.g.
substance,reaction,datasource) stored in the database, a property graph, which maintains links between different data objects (vertices), can be imposed later - All the data connected by links (edges) can be directly retrieved by traversals It is easy to follow links between the data objects ( e.g. in which reactions does this substance participate? which data source references are used for this data object? )
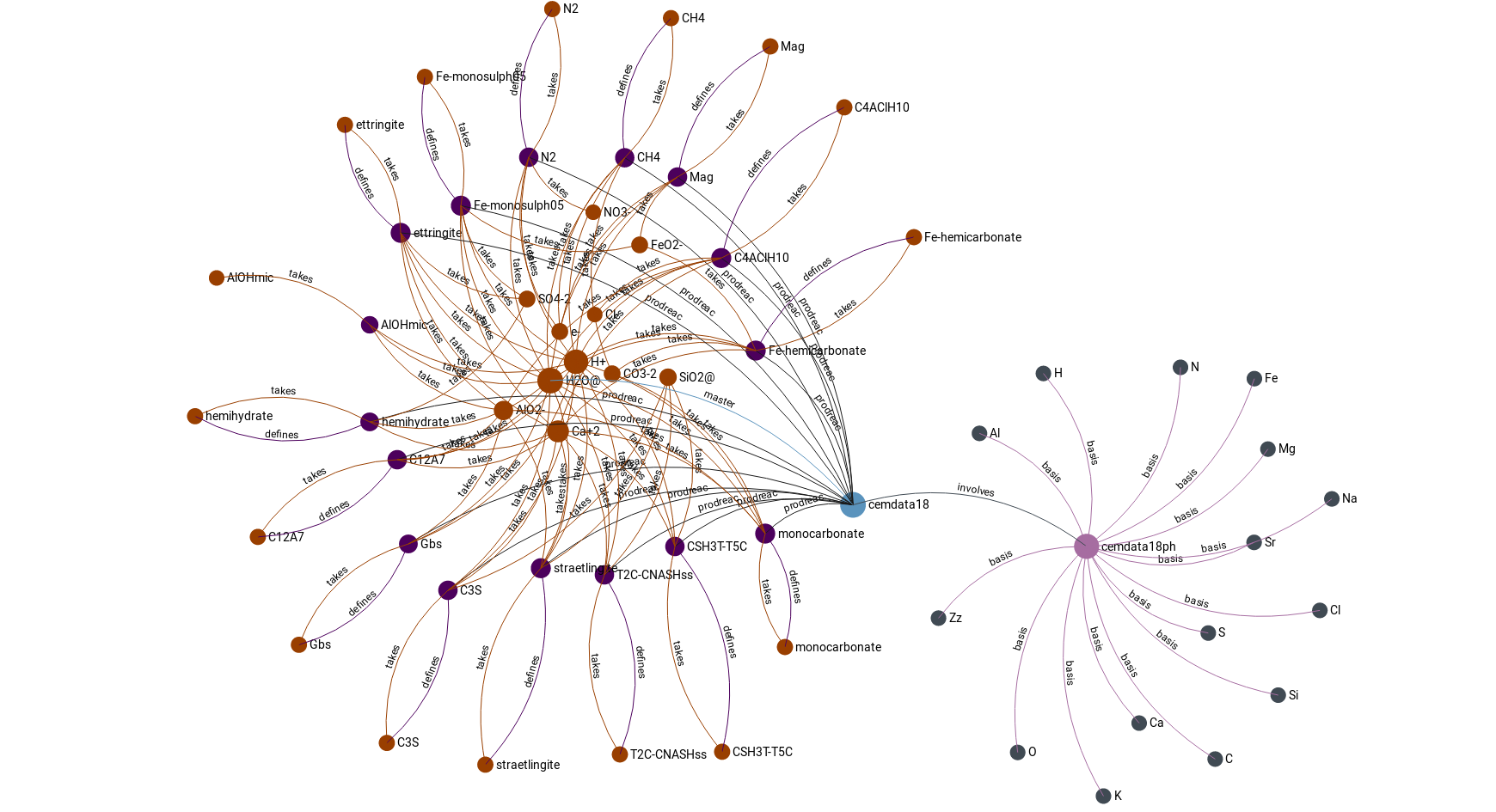
- ThermoHub can store both GEM (Gibbs Energy Minimization) substances based and LMA (Law of Mass Action) reactions based thermodynamic data sets
Data Source
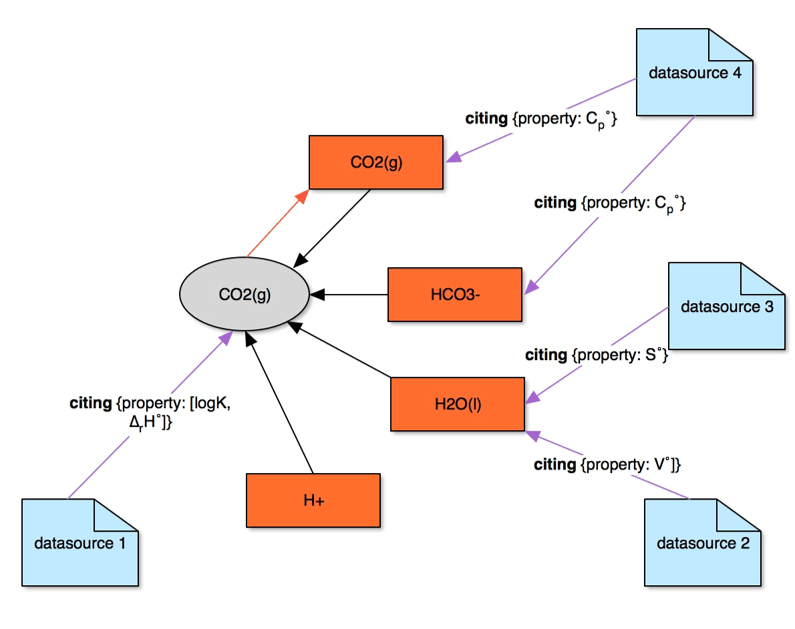
- All the data can be connected through graph links of type “
citing” to the appropriate bibliography references (“datasources”) - Whole data vertex, any property item, parameter, or coefficient in a list can be linked to a
datasource - Error estimates can be entered for each property value, together with the error types. These can also be traced via links to
datasources - Graph links can contain comments
- Graph layout makes it easy to ask questions like:
- From which data source(s) were certain data derived?
- What other data were derived from a given data source?
DataSource records can be imported from BIB, RIS and other popular formats.
ThermoDataSets
What normally researches would refer to as a thermodynamic database (e.g. slop98), here a ThermoDataSet, is a collection of thermodynamic data for substances and/or reactions put together by different scientists with different degrees of accuracy and internal consistently.
Examples of ThermoDataSets
Some ThermoDataSets available in ThermoHub. ThermoHub is continuously updated with new ThermoDataSets!
- psinagra-12-071 – waste disposal
- slop98-inorganic and slop98-organic2 – aqueous geothermal (revised SUPCRT92)
- cemdata183 – suitable for cement systems
- heracles4 – modeling of U and fission products
- mines165 – modeling magmatic-hydrothermal ore forming processes
- aq176 – modeling fluid rock interaction at hydrothermal conditions
- slop167 – aqueous geothermal (organic and inorganic)
New Databases (hub_ord):
- PSI/Nagra TDB20201 – update to the psinagra-12-07 for waste disposal, radionuclide, environmental geochemistry
- ThermoChimie12a8 – for waste disposal, radionuclide, environmental geochemistry
- MINES235 – for fluid rock interaction magmatic-hydrothermal ore forming processes
- NASA029 – NASA Glenn Coefficients for Calculating Thermodynamic Properties of Individual Species
- CODATA10 – CODATA Key Values for Thermodynamics
-
Hummel, W. & Thoenen, T. (2023): The PSI Chemical Thermodynamic Database 2020. Nagra Technical Report, NTB 21-03.
-
Johnson, J.W., Oelkers, E.H. and Helgeson, H.C. (1992) SUPCRT92: A software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous species, and reactions from 1 to 5000 bar and 0 to 1000°C. Computers & Geosciences 18, 899-947.
Shock, E.L., Sassani, D.C., Willis, M. and Sverjensky, D.A. (1997) Inorganic species in geologic fluids: Correlations among standard molal thermodynamic properties of aqueous ions and hydroxide complexes. Geochimica et Cosmochimica Acta 61, 907-950.
Sverjensky, D., Shock, E. and Helgeson, H. (1997) Prediction of the thermodynamic properties of aqueous metal complexes to 1000 C and 5 kb. Geochimica et Cosmochimica Acta 61, 1359-1412. ↩
-
Lothenbach, B., Kulik, D., Matschei, T., Balonis, M., Baquerizo, L., Dilnesa, B.Z., Miron, D.G., Myers, R. (2019) Cemdata18: A chemical thermodynamic database for hydrated Portland cements and alkali-activated materials Cement and Concrete Research, 115,472-506
-
Gysi, A.P., Hurtig, N.C., Pan, R., Miron, G.D., and Kulik, D.A., 2023, MINES thermodynamic database, New Mexico Bureau of Geology and Mineral Resources, version 23, https://doi.org/10.58799/mines-tdb
-
Miron, G.D., Wagner, T., Kulik, D.A. and Lothenbach, B. (2017) An internally consistent thermodynamic dataset for aqueous species in the system Ca-Mg-Na-K-Al-Si-O-H-C-Cl to 800 °C and 5 kbar. American Journal of Science 317, 755-806. ↩
-
converted from slop16.dat (last updated: 12.Mar.19 GEOPIG), https://gitlab.com/ENKI-portal/geopig/blob/master/slop/slop16.dat ↩
-
Eric Giffaut, Mireia Grivé, Philippe Blanc, Philippe Vieillard, Elisenda Colàs, Hélène Gailhanou, Stéphane Gaboreau, Nicolas Marty, Benoit Madé, Lara Duro. Andra thermodynamic database for performance assessment: ThermoChimie. Applied Geochemistr 49 (2014) 225-236.
-
Cox, J. D., Wagman, D. D., and Medvedev, V. A., CODATA Key Values for Thermodynamics, Hemisphere Publishing Corp., New York, 1989.
